-

Molekular sëlleren Jz-Zm3
-

Molekular sëllegen Jz-Zms4
-

Molekular sëllegen Jz-Zms5
-

Molekular sëlleren Jz-Zms9
-

Molekulär Seif Pulder Jz-zt
-

Molekular sëlleren Jz-Az
- Broessdatsch
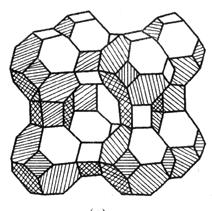
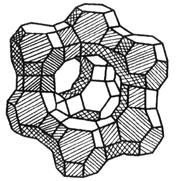
- Molekular Seife (och bekannt als synthetesch Zolite) ass e silizéierten Kristall. It is a basic skeleton structure composed of silicon aluminate, with metal cations (such as Na +, K +, Ca2 +, etc.) to balance the excess negative charge in the crystal. Den Typ vu Molekular Seifal ass haaptsächlech an engem Typ opgedeelt, X Typ an Y Typ no senger Kristallstruktur.
| Chemesch Formula vun der Zolite Zellen | Mx / n [(al.2) x (sio.2) Y] wh.2O. |
| Mx / n. | CARGIOUN ION, HOLD DE CRIYSTAL Elektresch neutral |
| (Alo2) x (Sio2) y | De Skelett vun der Zolite Kristaller, mat verschiddene Formen vu Lächer a Kanäl |
| H2o | Kierperlech adsorbed Waasserdamp |
| FONTassementer | Multiple Adsorption an Desorptioun kann ausgefouert ginn |
| Typ Amolekular Seifal | | D'Haaptkomponent vum Typ e Molekular Silie ass Silicon Aluminat. The main crystal hole is octaring structure.The aperture of the main crystal aperture is 4Å(1Å=10-10m), known as type 4A (also known as type A) molecular sieve; Exchange the Ca2 + for the Na + in the 4A molecular sieve, forming an aperture of 5A, namely a 5A type (aka calcium A) molecular sieve;K+ for a 4A molecular sieve, forming an aperture of 3A, namely a 3A (aka potassium A) molecular sieve. |
| Typ X Molekular Seif |
| The main component of X molecular sieve is silicon aluminate, the main crystal hole is twelve element ring structure.Different crystal structure form a molecular sieve crystal with a aperture of 9-10 A, called 13X (also known as sodium X type) molecular sieve;Ca2 + exchanged for Na + in a 13X molecular sieve, forming a molecular sieve crystal with a aperture vun 8-9 A, huet en 10x (och bekannt als Kalzium X) Molekular Seifal. |
- D'Applikatioun